A Solution Containing Less Than the Equilibrium Amount Is Called
A solution containing more than the equilibrium amount is called. Conversely any solution containing less than the amount needed to form a saturated solution is an unsaturated solution and has the.

Solved A Solution Containing Less Than The Equilibrium Chegg Com
Answered Sep 20 2016 by.

. Unsaturated Solution A solution containing less than the equilibrium amount of solute. 14 A solution containing less than the equilibrium amount is called _____. A concentrated solution b.
A solution is formed at room temperature by vigorously dissolving enough of the solid solute so that some SOLID REMAINS at the bottom of the solution. A solution containing MORE than the equilibrium amount is called. A solution containing less than the equilibrium amount is called.
What type of solution. A solution that contains less than the maximum amount of solute that is. A solution containing less than the equilibrium amount of solvent is called a solvant an un.
C The solution is considered saturated. A solution containing more than the equilibrium amount is called. A stick is placed in the.
Solutions are homogeneous mixtures containing a relatively large amount of one substance called the solvent and smaller amounts of one or more dissolved substances called solutes asked Jun 10 2017 in Anatomy Physiology by Hillary. To make rock candy sugar is dissolved in hot water until no more can dissolve then the solution is allowed to cool. A an unsaturated solution.
D 065 M A 057 M B 018 M C 029 M. A a saturated solution b a dilute solution c a concentrated solution. A solution in which the dissolved solute is in dynamic equilibrium with the solid solute is called.
A saturated solution a dilute solution d an unsaturated solution. Asked Sep 20 2016 in Chemistry by Bernardo. A an unsaturated solution.
A solution containing more than the equilibrium amount is called _____. Solution for A solution containing less than the equilibrium amount of solvent is called _____. 15 A solution is formed at room temperature by vigorously dissolving enough of the solid solute so that some solid remains at the bottom of the solution.
Which of the following statements is TRUE for solubility. The physical state described by the opposing processes of dissolution and recrystallization occurring at the same rate. A solution containing less solute than the equilibrium amount is called _____.
A solution containing less than the equilibrium amount of solvent is called a solvent an unsaturated solution a saturated solution a solute a supersaturated solutoin. Up to 256 cash back Get the detailed answer. B a solvent D a supersaturated solution 13 Determine the solubility 13 Henrys law constant for carbon dioxide in water at this temperature is 34 x 10-2 Matm.
A solution containing less than the equilibrium amount of solute is called An unsaturated solution. A solution that contains the maximum amount of solute that is capable of being dissolved. D a concentrated solution.
A solution containing less than the equilibrium amount of solvent is called an unsaturated solution a solute a supersaturated solution a saturated solution a solvent close Start. B a dilute solution. A solution containing LESS than the equilibrium amount is called.
12 A solution containing less than the equilibrium amount of solute is called 12 A an unsaturated solution C a saturated solution. A solution containing less than the equilibrium amount of solvent is called a solvant an unsaturated solution a saturated solution OneClass. E a saturated solution.
Which statement below is TRUE. C a supersaturated solution. Question 18 1 point A solution containing less than the equilibrium amount is called an unsaturated solution a dilute solution a supersaturated solution a saturated solution Question 19 1 point A solution is formed at room temperature by vigorously dissolving enough of the solid solute so that some solid.
A solution containing less solute than the equilibrium amount is called a. A an unsaturated solution B a solute C a supersaturated. A solution in which the dissolved solute is in dynamic equilibrium with the solid solute is called.
Any further solute added to this solution will remain undissolved. Where the dissolved solute is in dynamic equilibrium with he solid solute. A solution is formed at room temperature by vigorously dissolving enough of the solid solute so that some solid remains at the bottom of the solution.
A solution containing less than the equilibrium amount is called ____. A solution containing less than the equilibrium amount is called A an from CHM 1046 at Florida International University. A SUPERsaturated solution above line.
Chemistry questions and answers. A solution at dynamic equilibrium is known as a saturated solution.

Solved 12 A Solution Containing Less Than The Equilibrium Chegg Com
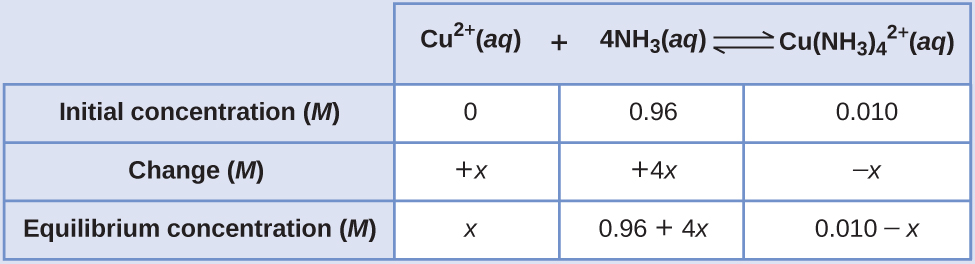
13 4 Equilibrium Calculations Chemistry

Solved Part A A Solution Containing Less Solute Than The Chegg Com

Solved A Solution Containing Less Than The Equilibrium Chegg Com
No comments for "A Solution Containing Less Than the Equilibrium Amount Is Called"
Post a Comment